[AUTHENTICITY CERTIFIED: Text version below transcribed directly from audio.]
Thank you very much.
I will be making some forward-looking statements since “future” is in the title of my talk. It doesn’t necessarily mean I’m advocating them or that I am optimistic. It means that in order to do engineering long-term planning, you need to think about -- and cautionary tales are in order -- like building a cathedral, you might have to plan a century in advance.
This is my conflict of interest slide. I’m highly conflicted.
But it’s also this: It’s a set of institutions, both non-profit and for profit, that help us bring our technology into reality, into daily life.

These are some of the topics we’ll talk [about], mainly the top three, which is this idea of synthetic versus natural, prediction, and where we stand in terms of human genome engineering. We have a love affair with the idea of the “natural” even though we as a species are about as unnatural as you can imagine.

I’ll mention four diseases. There is diabetes and heart disease to which we succumb due to natural compounds such as sucrose and cholesterol.

And the sourc[es] of carcinogens are robust in nature. We get on the order of 1500 milligrams a day of plant toxins and pesticides that are listed as rodent carcinogens. That’s the cancer side. And the infectious disease side is there are many instances where infectious agents are spread through vegetables and meats.

We have a huge difference of intention and obsession with risks. The risks of genetically modified foods are not well-documented. The risks of driving a car are very well-documented, 1.2 million deaths a year. My laboratory and my obsession is about safety and building/engineering safety. It’s not just a matter of saying we want the world to be safer; we have to create technology. And here are some of technologies that we use for automobiles. It doesn’t sound like we’ve done our job entirely if there are still 1.2 million deaths a year. But these are [the] sort of things that inspire us to do better in a variety of engineering fields, including genomics engineering which is what we are talking about here.

We have standards in testing in biology. Increasingly, we have these redundant systems and isolation that we’re using: physical, genetic and metabolic. I have advocated that we do extensive education, licensing and surveillance since 2004; in particular, in the field of synthetic biology. Since the technology is getting exponentially less expensive, more able to engage the public through “do-it-yourself biology,” I felt then and feel now that we need to have better and better tools for tracking the dissemination of the information, the chemicals and the instruments.
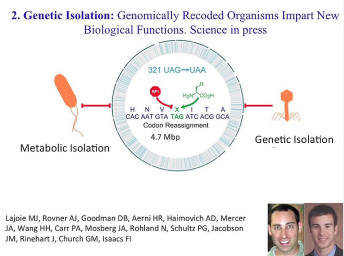
Here’s just a snapshot from the trenches in the laboratory. We’re trying to address what it is that people want when they say that they want genetically engineered organisms that do not spread in the wild. This is one of the many critiques of genetically modified organisms. And this is one attempt that we’ve recently published where we make them genetically isolated, metabolically isolated, even if they -- whether or not they’re physically isolated from the environment, they cannot exchange genetic material successfully.

So let’s talk about prediction.

These were forward-looking statements where The New York Times -- I had nothing against The New York Times, Amy -- but back in 1903 they made the modest prediction that there would be 1 to 10 million years, a nice, broad range, for a flying machine. And even Wilbur Wright, who was much closer to the event than the New York Times perhaps, was off by about 48 years in his estimate. And so we make such mistakes -- and even within my fields of computing and biology.
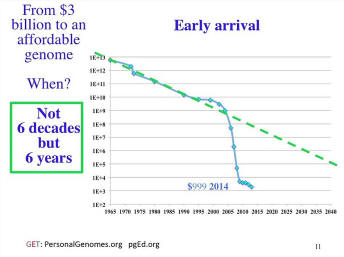
We have these two trend lines, sometimes called Moore’s law. It’s not really a law; it’s a trend. It could deviate from the line at any point. The blue line which is various biological things, sequencing and synthesis, reading and writing DNA -- it followed the electronics Moore’s law line at about 1.5 fold per year for many years and so it seemed like, “Wow, this must really be a law that’s totally general, biology and electronics.” But then we blew it and we went way off the reservation and it took not six decades to bring us down to an affordable genome, but more like six years. So we’re down around a thousand dollar cost. We don’t yet have a thousand dollars that you can go out and individually get genomes, but it’s at bulk rate that’s where we are right now, which I think is cost effective today, for a variety of things.
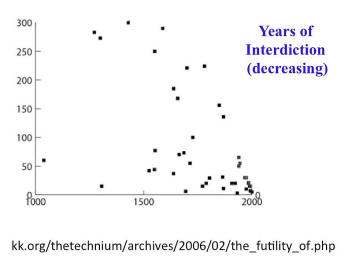
Kevin Kelley documented how long it takes for new technologies in general, this includes crossbows and so forth, to be banned and then the ban to be released. It’s almost inevitable that technologies, really dangerous technologies like railroads and automobiles, would be controversial. But the time that it’s taking before they become uncontroversial is shrinking. This isn’t to say that we shouldn’t have controversy in discussion. It’s just where are we going. It’s to describe where we’re going.
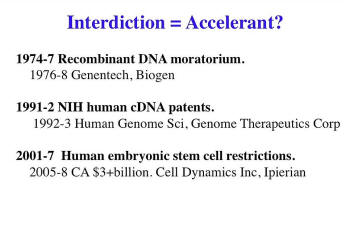
Now these are three types of technologies that I was intimately involved in. Each one of them had some sort of controversy and some sort of response. So, controversy being the bold one, and then the response; and I’m making the very casual, not well-documented argument that in some cases these were not deterrents to progress. They were good conversations that, if anything, accelerated the field. So, the Recombinant DNA was sort of 1974, got a lot of attention -- covers of Time magazine and things like that; and almost instantly, Genentech, Biogen, Amgen and a variety of other Biotechs got investors, because investors said, “Oh, it’s controversial, there must be something here. Let’s take a closer look.”
A similar thing happened in 1991, where the NIH actually started the controversy by patenting a lot of cDNAs that Craig Venter’s group had done back when he had a small NIH lab.1 And the lawyers had just said, “This is what we do. We patent things that come out of the NIH labs.” And then people said, “Well, you can’t patent cDNAs. You haven’t added much value to it. They're natural pieces of DNA.” We now see that cDNAs are patentable, but I would argue that there wasn’t much value added to these. And the controversy resulted in a great deal of attention and almost immediately that research moved out of NIH to Human Genome Sciences and Genome Therapeutics, a company that I was helping out getting early DNA sequencing going.
Human embryonic stem cell[s]. There are lots of restrictions in the United States, especially. This resulted in -- I can’t say for sure but I doubt that California would've coughed up quite that much money if there had not been that much controversy and frustration among the California biologists -- and they sort of saw this as an opportunity to redo -- to do another Silicon Valley, to make "Stem Cell Valley," and then there are these various successes.
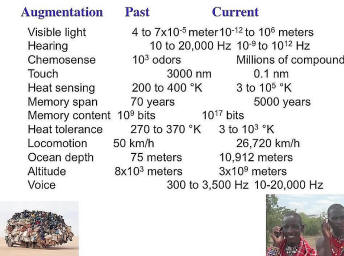
Now augmentation is something [that] often comes up with genetics. And we all put aside all the augmentations we’ve made that make us unnatural as a species. We’re an extremely unnatural species. We have -- ignore all the numbers; they’re a little bit out of alignment, but the point is we have senses that go over much broader range than they used to; our ability to access/retrieve information is vast because [of] our computers and our cell phones (access to them); we can go at vast speeds into space, into the depths of the ocean, and so forth. And you can see, many of these technologies take a fairly short time, not just through the interdiction process but just through the cost curve, to get broadly distributed -- not always in the optimal way, obviously here is transportation and cell phones, but the point is they can have a huge impact and they can be commoditized worldwide, where you can have microfinancing, and where people get huge benefit from fairly modest access to electronics.
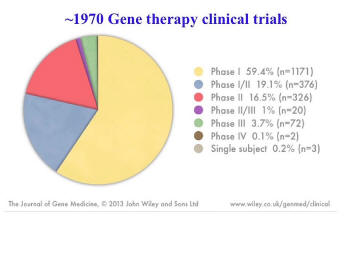
So, are we genetically engineering human beings? And the answer is, “definitely.” We’ve been doing it for quite a while.
And this is a summary of where we stand in terms of gene therapy trials; which is one of various ways that we are modifying human beings. These are, for the most part, adults. There are some of these [that] are children. And there are Phase 1, 2 and 3 clinical trials. There’s one that’s been approved in Europe called Glybera. It is, right now, the most expensive pharmaceutical ever, at 1.6 million dollars per dose. But as orphan drugs go, that’s actually about right because you only need one dose, and it shows a general acceptance of this kind of human genetic modification in Europe, which has a reputation for being skeptical about such things. And it has to do with the seriousness of the diseases that are being treated. So, the key here is not so much ethics as safety and efficacy; it’s the standard by which the Food and Drug Administration and the Environmental Protection Agency go by, if it’s safe and very effective and is addressing the real problem.

Now, the most extreme and interesting form of those 2,000 or so genetic therapies that are in clinical trials, to me, is this highly targeted gene therapy. Most of those on that previous slide are adding a gene, compensating for [a] missing gene, a complete loss of function. And so you can add it pretty much anywhere. You can integrate it randomly, which has certain risks associated, or you can “not integrate” it, but it’s adding. But you can also subtract or you can specifically modify. You can use endonucleases that are programmable. That is to say, you can target them to find a needle in a hay stack, that one base pair out of six billion in your genome that you want to change.
And in this case, this the earliest of these that’s making it through clinical trials. Sangamo is in sort of Phase 1, Phase 2 clinical trials. And you delete or damage both copies of CCR5 gene and you end up in -- with the T-cells from the person. You can take them out of the person, manipulate them, and put them back in. That means there’ll be no graft rejection, no need for immunosuppressants. And they have now, the ones that have taken up this zinc finger nuclease, this specific targeted in the nuclease, those cells -- those T-cells -- that have been doubly knocked out of the CCR5 gene are now resistant to the AIDS virus. And this could be done in patients that already have full blown AIDS. And so again, this is a very promising for very severe diseases.
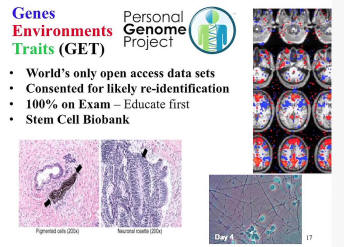
We have a project to study personalized medicine in its broadest sense, ranging from carrier status, new-born screening -- all aspects of modern human genetics that works quite well to new personalized medicines that include microbiomics2 and complicated multi-factors. To get this multi-factor data set, this large data set about individual people, not about cohorts but really N-of-one, lots of N-of-one studies, we have this open-access data that requires consent that shows that people really know what they’re getting into in terms of the possibility -- the probability that their data will be publicly available. We have stem cells for many of these individuals and a variety of tests that can be done.
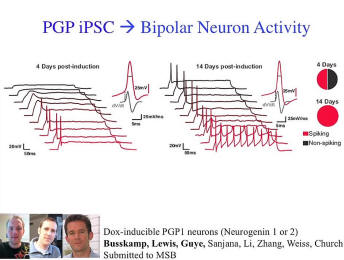
The sort of stem cells that come out of this are a little piece of the BRAIN Initiative -- that you heard just a moment ago -- where you can take these stem cells, reprogram them into neurons, and then they can have this kind of neurophysiology and it could be done in very short developmental time frames. So we’re getting to the point -- another kind of “brave new world” issue -- where we can not only manipulate human genomes but we can manipulate human brains. We can either program them in situ with these innovative neurotechnologies, or we can build brains outside of the body and get this kind of spiking behavior on neural nets.

This BRAIN Initiative was partially instigated by this group of six people that I was part of in 2012. And it’s really remarkable how quickly it went from that semi obscure, forward-looking piece in Neuron (the journal) to an announcement by Barack Obama that we had a BRAIN Initiative. I mentioned nucleases that you can target -- you can program to find a needle in a hay stack of the human genome or other genomes.
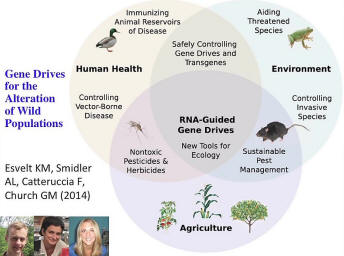
There are a variety of ways of making these nucleases, very specifically, but they can be targeted not just in the human genome, but into wild genomes. We know that we’re releasing genetically modified organisms into the wild for crops, for plants. There are a few animals that are being released in the wild; for example Oxitec is releasing Aedes aegypti mosquitoes which have been engineered to be essentially like males that have been sterilized by radiation, but in this case they been sterilized by genetics. That seems like a fairly safe thing since the males don’t suck blood and they are sterile. It’s a special case but you can see that the communities are warming up to the idea of releasing plants and animals into the environment.
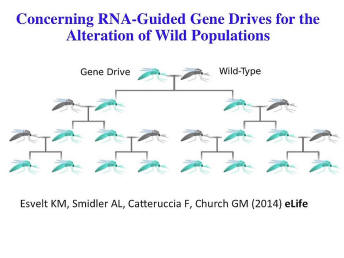
This would be the most extreme form of that and as a consequence Kevin Esvelt, Andie Smidler, Flaminia Catteruccia and I published three papers very recently laying out this new form of gene drives. The basic idea goes back to Austin Burt in 2003 at least. But this is a CRISPR-based gene drive that’s so much easier, probably, to do and might be something worthy of a cautionary tale -- something worthy of being very thoughtful about, because it is so enticing and because it is so easy to do that even individuals might be able to do it. So now is the time to talk about it before we go much further.
Now since we've published these papers, we’ve already now done it -- experiments on gene drives (this not published) in yeast -- we’ve done it in yeast because they have such a rapid generation time. This method is directed at sexual transmission where you can correct spread at a desired package through a wild population or, in this case, a laboratory population exponentially so all the offspring have it. And the experiment in yeast, it’s been close to a hundred percent. This is kind of the simple version of how it spreads [or it’s spread].
 This is decidedly not like the
Gregor Mendel peas that we saw a few
slides ago, in
Paul’s [Lombardo] talk.
This is decidedly not like the
Gregor Mendel peas that we saw a few
slides ago, in
Paul’s [Lombardo] talk.
This is something where, essentially, you introduce one or a few mosquitoes into the population that have the gene drive and it spreads exponentially to all of their offspring and all of their offspring’s subsequent matings until it is throughout the population; and it can spread packages such as resistance of the mosquito to malaria. So you’re not actually hurting the mosquito but you’re making them so they no longer can be vectors for malaria; or you could make it so that you only get transmission of the Y chromosome, in which case you eventually result in decrease in the mosquito population.
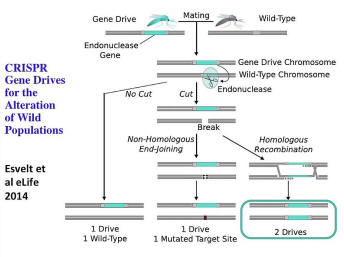
This is a little more technical of what's happening behind the scenes, but you basically have the blue cassette in the upper left that is on one chromosome in one specific position. So this is selfish DNA. It’s not DNA that is wildly replicating all over every chromosome -- hence, producing a huge burden on the mosquito or whatever species you’re dealing with, whether invasive or vector species. But it’s in one place, in one chromosome that you’ve decided is the best place. And then, when in the embryo, if it finds that same place in the chromosome from the other parent is different -- doesn’t have the cassette -- then we'll move the cassette over into that by making a cut -- makes a double strand break -- and the cassette moves into it; and now you either have both copies, (all the copies) of that particular chromosome looking the same, or perhaps you’ve mutated. Either way, all the offspring that survive have the cassette that you want.
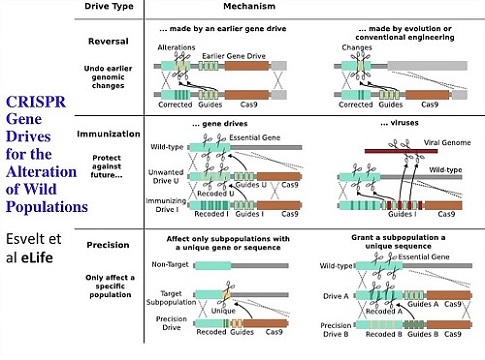
And forget the details of this, but the important thing is there are very sophisticated things you could do with this new technology -- this CRISPR technology -- that allows you to be very specific -- target sub-species -- so you have not only the species’ mating behavior and geographical barriers but you have this genomic barrier that you can engineer so that you can make sure that the cutting is efficient and specific and even sub-species specific. You can do immunization either against gene drives or against natural components like viruses; and you can even make reversal drives.
I will make the argument that we are poorly adapted to our current environment. I mean, we did not evolve to sit all day and be exposed to giant amounts of really tasty food. And there are various opportunities that we have which I would -- certainly [they're] not eugenics and they’re not even augmentation in that we’re dealing with diseases of civilization. They are augmentations in a certain sense in that we're putting things into our medicines or our gene therapies that deal with things we wouldn’t have had to deal with in the past, but we respond to these in an emergency attitude because they are life‑threatening.

This changes again when we go into new environments and our civilization keeps pushing itself into new places such as space. In space, we have problems with osteoporosis due to the lack of gravity. We have extreme radiation problems. Now some of these have corresponding problems on earth. There are problems with the inner ear. There are problems with special stresses that occur there and so forth.

When we deal with these situations we worry about multigenics. That is to say, how can we possibly understand something as complicated as height when there are now 700 genes that affect it and they have very tiny affect sizes? What’s important -- and you find it from projects like the Personal Genome Project, where you can correlate traits with genomes -- is looking at the extremes. We talked about the bell curve. This is a different kind of bell curve where you look way out at the shortest and the tallest people as an example of many different traits, both medical and non-medical. And you see, out at those extremes, you get things that are called epistatic genes -- variants that are so powerful that they dominate, epistatically, all of those small effects, including some small environmental effects, some small genetic effects, and these suggest cures as well, or treatments that will influence these very complex genetic and environmental traits.

So if you give growth hormone to a young person, maybe even at an age where growth should be stopping, growth will continue and their stature will approach whatever stature goal they’ve had. So this is an example of something where you can have what looks like a very complex genetics with fairly simple consequences -- not that anything about medicine is simple.

You will often have cases where you have one person on the planet at any given moment, where you’ve learned that you don’t have huge statistics here. You have a cohort size of one. Nevertheless, you can prove causality by making animal models in some cases; some cases making human in vitro organ models. And these you can consider rare protective alleles.3 These are things like, in this case, myostatin. You get a double “null,” meaning missing both copies. And more or less at birth this child had a high musculature, as you can see reflected in the animal models.
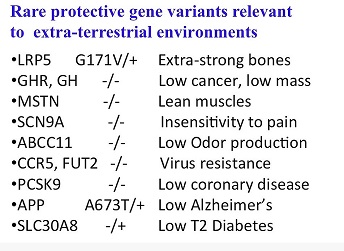
And there are many of these. None of these are unmitigated -- protective variants that have no down side -- but they have interesting possibilities. Some of these I mentioned in the context of imagining that they could be useful to deal with diseases of civilization or new environments such as space. So, for example, there are LRP variants. This is a heterozygote, which have extra strong bones -- or I mentioned growth hormone. You might want to have smaller people in space; you have insensitivity to pain; you have -- odor might be a problem in space; you can have multi-virus resistance, low coronary disease, and so forth. These are floating around our population. Many of them, minus over minuses, are double nulls. These are genes that are conserved among all animals but they’re missing in [some] human beings and they’re not only alive, in many cases they are thriving.

A possibly extreme example of this are people who live past 110 years. This slide is not an advocacy of smoking and drinking to excess, but the point is it’s probably not solely that they have a great environment. They may actually have some exceptional genes and so we’re studying their genomes and trying to test hypotheses about them.

Wrapping up here, as we go into space we need to study biospheres -- how our environment interacts with our genetics -- in more detail.
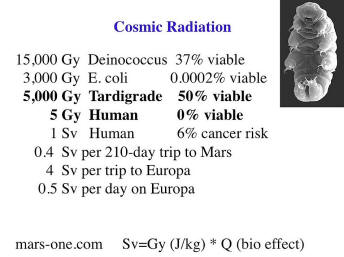
I’ve already mentioned cosmic radiation.

There are organisms that are very distantly related to us, such are radiation resistant, and you might say how irrelevant can that possibly be? How many mutations will that take? Well it turns out you can change with as few as four mutations an organism to be a hundred thousand times more radiation resistant. Whether this applies to humans is still unknown.

And I’m just going to end this very playful and speculative slide. This is a typical operating room. We will have to make a decision, as we go into new environments outside of earth, whether we want to drag along with us all our pathogens. We can or we can’t -- it’s up to us -- but I consider that part of genome engineering is how we interact with the huge part of our genome which is our microbiome. And I’ve mentioned that there are people that have insensitivity to pain. You can engineer this such that it could be turned on and off, and so rather than going into general anesthesia with its risks, or opiate-type analgesics, you could have something like this.
So I thank you, and time for questions, I hope.
1 The National Institute of Neurological Disorders and Stroke (NINDS), a direct descendant of the Institute of Neurological and Communicative Disorders and Stroke (NINCDS) at the National Institutes of Health. More on Dr. Venter and his work here. Brief synopsis of the initial NIH gene sequencing technique patent filings here and, a more detailed background review and technical analysis here.
2 See also, The Human Microbiome Project
3 Class of of gene modifiers that can suppress disease in otherwise susceptible individuals (Source: http://www.ncbi.nlm.nih.gov/pubmed/12787792).
Speaker Note: Professor George M. Church is the Robert Winthrop Professor of Genetics at Harvard Medical School and Professor of Health Sciences and Technology at Harvard and MIT, and has been a founding member of the Wyss Institute for Biologically Inspired Engineering at Harvard (Wikipedia.org).
Transcription Note: The foregoing as delivered transcript has been edited for content accuracy and continuity of style. Originally delivered substantive content preserved. I am grateful to Professor Church for providing editorial contributions to the above transcription. Principal transcription work by South Transcription Unlimited, Inc. | www.southtranscription.com | info@southtranscription.com | (+63) 920.921.8709. Supplementary transcription work and editorial oversight by Michael E. Eidenmuller.
Page Updated: 11/23/23
U.S. Copyright Status: Text & Images = Restricted.
